Thursday, December 6, 2012
I am in the top 20 Midwifery Blogs
http://www.kwikmed.org/20-of-the-best-midwife-blogs-in-2012/
Wednesday, September 5, 2012
NZCOM conference August 2012
I was asked if I would make my presentation available and so I have loaded it to slideshare and I am sharing it here.
The topic is around communities of practice, and the learning that can happen when midwives are able to engage with a variety or different practice groups. I am also interested in learning that happens when individuals or groups have differing opinions. While it is lovely to agree with people this does not always lead to improved understanding and new learning. When someone has a different opinion this starts us to wonder and is often the stimulus to either reinforce and be strong in our own understanding or perhaps to move our thinking and develop a whole new way of looking at things. This is topic I find interesting. I think it has implications for how health professionals can work together and collaborate.
Wednesday, August 15, 2012
Unethical marketing practices by Formula Manufacturers
""These samples have been provided under the provisions of Article 7.3 of the Intfant Nutrition Council Code of Practice for the Marketing if Infant Formula (I will leave you to read that from the code linked here). Where the suitability of a product is being assesed for an individual infant, the professionsal evaluation will always include a follow-up meeting with the mother of the infant; The product samples of infant formula products should be kept out of public view;
""These samples are not to be resold of taken for personal use by healthcare professionals or other staff;
If Pfizer grants this request for infant formula units, Pfizer may make inquiries of our staff periodically and may request reasonable documentation in support of the number of formula units requested, or actually recieved, by my institution or Healthcare System;
"Professional evaluation" applie to one or all of the following situations: *Analysis or products (ingredients, taste, nutritional profile);
*Trial preparation and mixing of infant formula products (includes preparation and mixing instructions to mothers);
*Investigation or development projects, using sound methodology and involveing a numver of infants;
*A thorough assessement of the suitability of a product for the individual infant, including acceptance by the infant, when mothers have made the informed choice to use infant formula.""
Wednesday, April 18, 2012
Letter to the editorial board of the British Journal of Midwifery.
You ask how in particular I feel the code is being breached and suggest that Nestle are a particular case in point, suggesting that other formula manufacturers are above reproach and therefore should not be considered in the same category as Nestle. From my very brief sojourn into the situation in the UK at present I can see that Cow and Gate are very from above reproach in the promotion of their products in the UK. Within this report from the baby feeding law group there are several instances where Cow and Gate and Aptamil, who are affiliated with Danone have been identified as not complying with the Code. I myself found one image on Flickr, taken in 2011 where Cow and Gate advertising on a baby change mat, in a shopping mall change room, does not appear to comply with that companies requirements under the Code. Where is the information on that breast is best? While it is advertising follow on formula and not newborn formula the distinction is not clear. The impression is that Cow and Gate produces health active beautiful babies. The British Journal of Midwifery should not associate itself with a company who uses these tactics to promote its product in breach of the Code.
As Jane Sandall (2008) describes, the midwife – woman relationship is a relationship of trust. Women expect their midwives to act in their best interest, not under the influence of advertising from a formula manufacturer. IN addition I would suggest midwives expect their journal to provide evidence based information and provide them with information which they can then use as a basis for sharing with women. Information on infant formula in midwifery journal should be scientific evidence based information not promotion of a particular brand of formula. I believe formula advertising anywhere in a midwifery journal is unacceptable.
Reference
Richter, J. (2005). Conflicts of interest and policy implementation. Reflections from the fields of health and infant feeding. Geneva, IBFAN- GIFA
Sandall, J. (2008).No such thing as a free lunch. Midwifery. 123 – 125.
Tuesday, April 17, 2012
Why not just give him a bottle? Infant formula company sponsorship of the midwifery profession

Way back in 1990 I organised a study day in Balclutha for rural midwifery. It was a huge success and we had about 90 midwives and other medical staff attend. We sought sponsorship from local businesses and had some trade stands. The local chemist was a sponsor and on their stand had some infant formula and related products. Shortly after the event we received a formal complaint from the monitoring body for the WHO/UNICEF code of marketing of breast milk substitutes, pointing out the many areas in which we were in breach of the code. The chemist received the same complaint. We were taken aback and it took the shine off what had been a great event. I became very familiar with the code and never committed a similar offence again.
Some time ago I stumbled on a documentary series from the Philipines on Youtube. It outlines the marketing strategies of infant formula companies in that country. This includes inducements to midwives with free samples and misinformation about the benefits of one or other infant formula. These midwives then promote the products to the women they are working with.
Recently I was made aware of the association between the British Journal of Midwifery and the infant formula company Cow and Gate in a midwifery awards scheme. When I investigated this further I found that Cow & Gate have a whole arm of the company devoted to providing research grants and educational support to midwives and other health professionals such as Health Visitors who have influence with women and are in a position to promote their product. While The company does not ask midwives to promote its product none the less midwives cannot help but be influenced by this sponsorship which is significant. This sponsorship is in fact not much different to that being given to the Philipine midwives in the documentary mentioned above. Cow & Gate In Practice was actually launched at the Royal College of Midwives in 1999. clearly the Code of Marketing of Breast Milk Substitutes has been interpreted quite differently in the UK to the interpretation in New Zealand. Or is it just that it has been largely ignored in the UK? Here is a summary of the code.
Through the In Practice website Cow & Gate promote their special hospital bottles of formula, plastered with their logo and full of information about the particular benefits of this formula.
I have challenged this promotional activity between the British Journal of Midwifery and the Cow and Gate milk company in an open forum and by email. However the In Practice website was launched at the Royal College of Midwives and the study days being delivered across the country are accredited professional development for the UK Nursing and Midwifery Council. It is therefore not just the British Journal of Midwifery who need to look to themselves and their relationships with infant formula companies. It goes much wider than that.
The International Board of Lactation Consultants had a similar issue with corporate sponsorship and have identified that this is a serious issue. They now have clear policies to avoid this.
I am just a New Zealand midwife. Although I was originally UK born and trained my working life has been here in New Zealand since 1981. It may be that I am too removed from practice in the UK to see these issues clearly from the perspective of practice in that country, or it may be that from this distance I am more able to see these things clearly. I have no wish to cause offence and apologise if I have done so. Am I totally mistaken in my opinion that there is a serious problem here? I value the thoughts and comments of others on this issue.
See this photo posted on Flickr by Sophie Passmore. It was taken on November 2011 and shows a baby change mat from a UK shopping centre changing room. I would have loved to use this here but it does not have an open licence.
Sunday, January 22, 2012
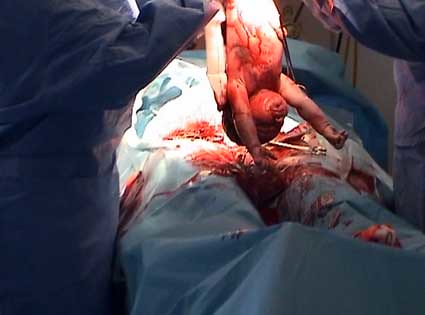
Casearean section. Midwives play their part
Increasingly babies are being born through an incision in their mothers abdomen. Sometimes this is after the mother has gone into labour but often without labour having been initiated at all. The baby is plucked without prior warning from familiar surroundings and brought into the world. The mother then has to recover from a major abdominal operation. Such surgery requires many weeks if not months of recovery. Why is it that this proceedure is increasingly required to bring babies into the world? On the 9th of February midwives in Otago are coming together to consider this issue to explore what is that we do that can might set women on this path. We are also looking at what we can do that can support women to avoid unnecessary caesarean section.
If you want more information about this call me through the 0800 Otago Polytechnic number and i will give you the phone number for registrations.